XBI testing Resistance this upcoming week!TICKER: $XBI
XBI will be testing daily resistance $97.00. The nasdaq biotech $IBB already broke its daily resistance so can XBI follow along?
Again, dividend distribution was this week and it has an impact on the price.
However, I would still be caution because the weeky RSI is pretty extended. RSI is @ 71ish and historic pullback RSI level is around 72.
Weekly time frame is extremely extended and a break of the higher low every weekly candlestick pattern will be the first indication of consolidation.
I am very interested in looking for a swing trade of LABD because of how extended the bulls are.
Biotech
$MNK Go timeAscending Wedge Reversal.
Volume is there and ready to go.
Generic opioid manufacturers have been beaten down recently over fear of a copious monetary fine, but MNK has an established diverse profile.
Do NOT miss this stock when it runs.
Break above $4.15 and were off
Long Term Target: $12.90
PCI Biotech $PCIB.OL top 10 hot nordic stocks @veckansaffarer
www.va.se
finansavisen.no
PCI Biotech OSL:PCIB is a biopharmaceutical company focusing on development and commercialisation of novel therapeutic solutions for improved treatment of cancer patients through its innovative photochemical internalisation (PCI) technology platform. PCI induces triggered endosomal release that can be used to unlock the true potential of a wide array of therapeutic modalities.
The company is applying PCI to three distinct anticancer paradigms:
• fimaCHEM – enhancement of chemotherapeutics for localised treatment of cancer
• fimaVACC – enhanced T-cell induction for therapeutic vaccination
• fimaNAc – nucleic acid therapeutics delivery
The company’s lead fimaCHEM programme consists of a pivotal clinical study with registration intent of Amphinex® in bile duct cancer, an orphan indication with a high-unmet need and without approved products. Clinical proof-of-concept has been achieved in the fimaVACC programme, which applies a unique mode of action to enhance the essential cytotoxic effect of therapeutic cancer vaccines. The fimaNAc programme utilises the triggered endosomal release to provide effective intracellular delivery of nucleic acids, such as mRNA and siRNA therapeutics, thereby addressing one of the major bottlenecks facing this emerging and promising class of therapeutics.
BerGenBio $BGBIO.OL rallies amid Norwegian biotech optimismBerGenBio OSL:BGBIO is a norwegian clinical-stage biopharmaceutical company focused on developing transformative drugs targeting AXL as a potential cornerstone of therapy for aggressive diseases, including immune-evasive, therapy resistant cancers.
The stock is currently in a short term uptrend that could provide a new test of important resistance, where a breakout to the upside could trigger a powerful buy signal.
Sponsored analyst summary:
www.trinitydelta.org
Twitter:
twitter.com
Investor Relations:
www.bergenbio.com
XBI potential pull back next weekTicker: $XBI
The biotech sector have been on absolute fire these couple of weeks. Check out the weekly chart with higher lows every candlestick.
The daily chart is getting toppy with somewhat of a double top at $96.61.
Break $94.08 this upcoming week and that will signal weekly consolidation. I will be scouting for a bearish play with LABD this upcoming week.
Weekly RSI also approach oversold so that means be cautious bulls.
Symmetrical Triangle BreakoutEYPT chart is building up for another run. Potential catalyst before year end with an sNDA for Short Duration Yutiq. accumulation has been slow and steady indicating EYPT oversold conditions.
Technical's:
weekly chart --> Harmonic Gartley (bounced off significant support level) and been in uptrend since.
Symmetrical Triangle/ Breakout setup has been known to act as a pre-courser to huge upside moves (40%+).
Bull flag building in the symmetrical triangle. . . Read the rest then check out the rest of our trade ideas HERE
Disclosure: I am long EYPT. I am not a financial advisor and this is not a buy/ sell recommendation but for education purposes only. Please do your homework before investing.
LONG OCXTraded in a 7 month range. Company plans on making 30million for the fiscal year 2021. 100mill marketcap so IT IS considered undervalued. TP at 2.40. TP 2 3.50. SL 1.60.
#VolatilityWatch Solid BioscencesBiotech buyouts have been very strong, especially with Gene therapy stocks as of late.
Bounced off Lower end of channel
Gartley pattern
bounced off 1.618 fib extension along with important reversal leg of Elliott Wave Theory.
if price holds inside the lower end of the channel there is a potential move back up and test the gap.
looking for nice $0.40+ swing from current level.
Disclosure: I am long SLDB. I may buy/ sell within the next 72 hours. I am not a financial adviser. Please do your homework before investing.
XBI could potentially break out hardTicker: $XBI
XBI needs to break 94.95 to break out hard. If we reject and form a 4 hour lower high compared to 94.95, WATCH OUT FOR THE 4H INVERSE HEAD AND SHOULDERS with support for 92.97 and 91.68!!!
Also watch for market correlation. If market is strong and hits ALL TIME HIGH, XBI could be in a great position to go long.
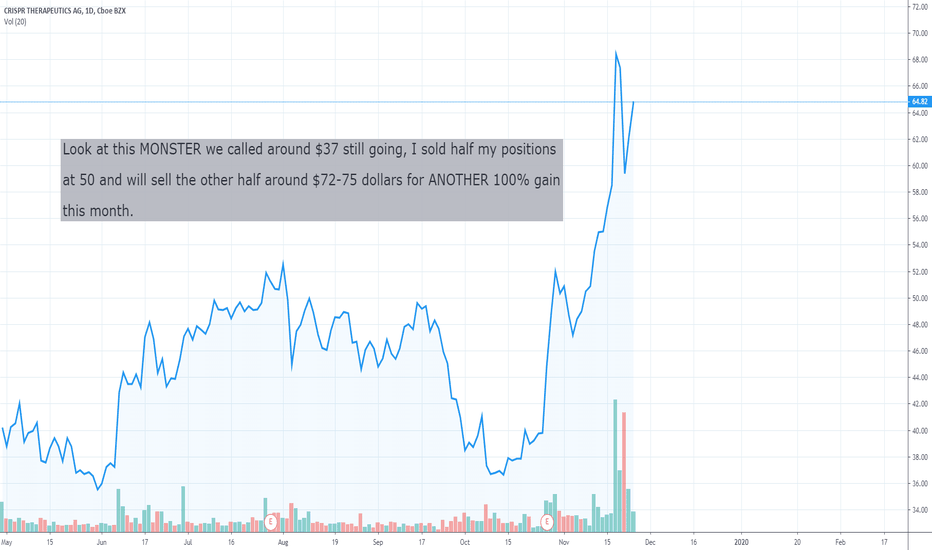
Buy the dip in SAVA based on strong Alzheimer's trial dataCassava Sciences (SAVA) just got some positive clinical trial data on their Alzheimer’s drug. The result caused one analyst firm to upgrade SAVA’s price target from $3.00 per share to $6.00 per share. SAVA’s got a 9.4/10 analyst summary score, with most analysts rating the stock a “buy.” I entered SAVA Friday morning at $2.16 per share. It’s already up almost 100% from its share price last month, but I think it could continue its run next week.
My Top Piece of shitThis is just garbage. Sitting here a bubbly parabolic ATH's. This is the #1 short on the planet right now. Overbought on the fucking weekly now in both RSi and MFI. The top is in.
Targovax $TRVX.OL clinical phase oncolytic virus valued 35 mUSDTargovax OSL:TRVX a clinical phase norwegian Biotech is currently at market cap NOK 327 million / 35 million USD.
Value inflection point very close as the company will need to raise funds in the very near future, which must either come from investors or from a possible partner deal.
PRESENTATION OF ONCOS-102 MELANOMA DATA AT SITC ANNUAL MEETING
www.healthcap.eu
Q3 report:
www.targovax.com
Q3 transcript:
finance.yahoo.com
Did the norwegian Biotech 3 year bear market come to an end?Oslo Stock Exchange info page:
www.oslobors.no
Oslo Cancer Cluster:
oslocancercluster.no
Radforsk:
radforsk.no
------------------------------------------
Photocure IR: OSL:PHO
photocure.com
Nordic Nanovector IR: OSL:NANO
www.nordicnanovector.com
PCI Biotech IR: OSL:PCIB
pcibiotech.no
BergenBio IR: OSL:BGBIO
www.bergenbio.com
Ultimovacs IR: OMXSTO:ULTIMOO
ultimovacs.com
Targovax IR: OSL:TRVX
www.targovax.com
Photocure $PHO.OL stock trade above resistance, close at 5yr hghRecent news by OSL:PHO :
Randomized phase 3 study in Denmark demonstrates significant advantage in Bladder Cancer for Hexvix/Cysview blue light cystoscopy over standard white light variant:
photocure.com
At Q3 2019 Photocure reported continued record growth in the US as well as upfront payments from Chinese partner Asieris:
photocure.com
Further improvements for US reimbursements for Cysview from january 2020:
photocure.com
Technical analysis:
Technical indicators sliding into high momentum and near overbought values at this price level, so any entry should be done with clear considerations of short term risks.